Diabetic Neuropathy: Advanced Pathophysiology, Clinical Manifestations and Contemporary Pain Management Strategies
Diabetic neuropathy represents one of the most prevalent and debilitating complications of diabetes mellitus, affecting approximately 50% of diabetic patients worldwide. This comprehensive review examines the intricate pathophysiological mechanisms underlying diabetic neuropathic pain, encompassing molecular pathways from hyperglycemia-induced metabolic dysregulation to complex neuroinflammatory cascades. We explore the clinical spectrum of manifestations, ranging from subtle sensory disturbances to severe neuropathic pain syndromes, and critically evaluate contemporary therapeutic strategies. Recent advances in understanding mitochondrial dysfunction, oxidative stress pathways, and central sensitization mechanisms have opened new avenues for targeted interventions. This review synthesizes current evidence on established treatments while highlighting emerging therapeutic modalities, including novel pharmacological combinations, regenerative approaches, and precision medicine strategies that promise to transform the management of this complex condition.
Introduction
Diabetic neuropathy encompasses a heterogeneous group of peripheral nerve disorders that constitute the most common complication of diabetes mellitus, affecting both type 1 and type 2 diabetic patients with increasing prevalence over time. The economic burden of diabetic neuropathy is substantial, with healthcare costs exceeding billions of dollars annually due to direct medical expenses and indirect costs associated with disability, reduced productivity, and decreased quality of life. Despite decades of research, the complete pathophysiological picture remains incompletely understood, contributing to the limited efficacy of current therapeutic interventions.
The complexity of diabetic neuropathy stems from its multifactorial etiology, involving intricate interactions between metabolic, vascular, inflammatory, and genetic factors. Hyperglycemia serves as the primary initiating factor, triggering a cascade of molecular events that ultimately result in structural and functional alterations of peripheral nerves. However, the relationship between glucose control and neuropathy progression is not straightforward, as evidenced by the persistence of neuropathic complications despite intensive glycemic management in many patients.
Recent advances in molecular biology and neuroimaging techniques have provided unprecedented insights into the pathogenesis of diabetic neuropathy, revealing novel therapeutic targets and biomarkers for early detection and monitoring. The emergence of precision medicine approaches promises to revolutionize treatment strategies by enabling personalized interventions based on individual patient characteristics, genetic profiles, and specific pathophysiological mechanisms.
Pathophysiological Mechanisms
Glucose-Induced Metabolic Perturbations
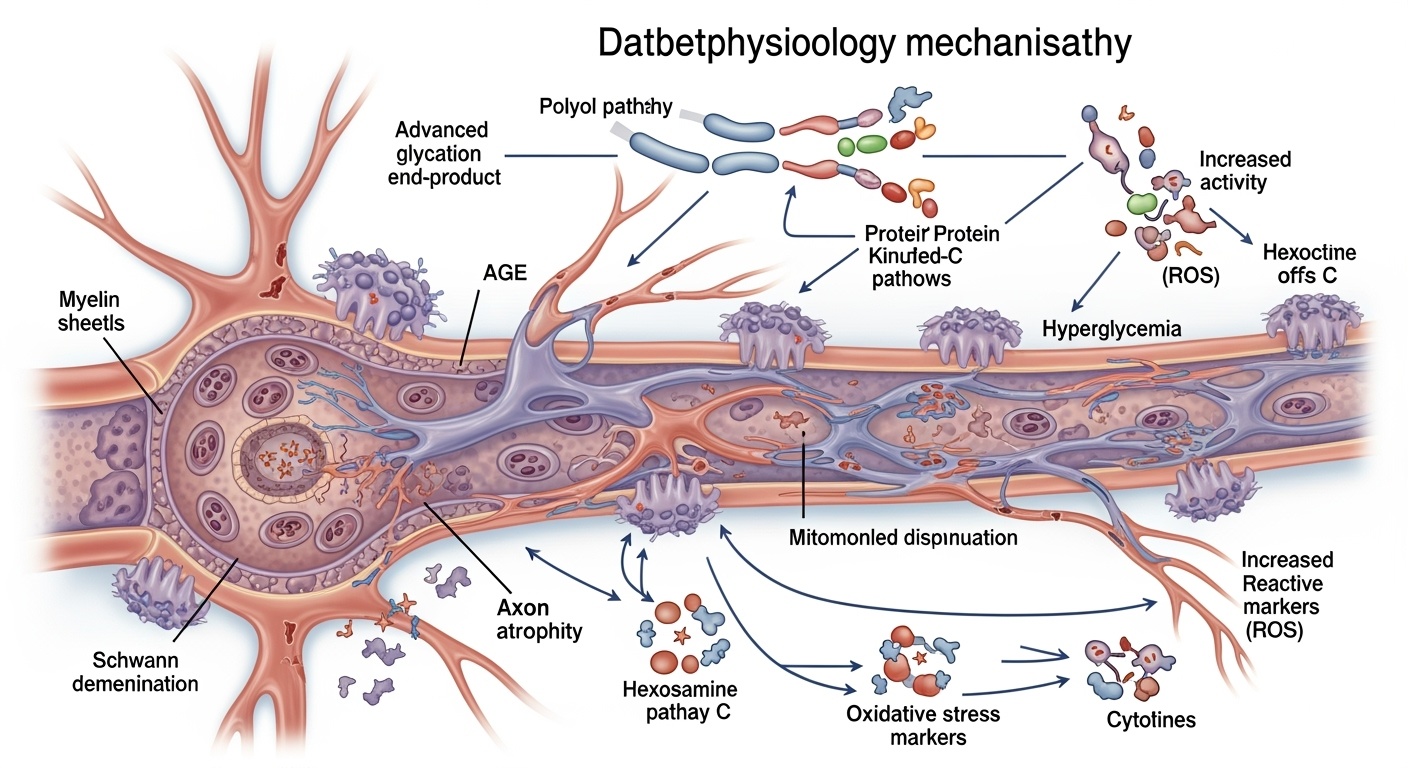
The pathogenesis of diabetic neuropathy begins with chronic hyperglycemia, which initiates multiple interconnected biochemical pathways that collectively contribute to nerve damage. The polyol pathway represents one of the earliest recognized mechanisms, wherein excess glucose is reduced to sorbitol by aldose reductase, subsequently oxidized to fructose by sorbitol dehydrogenase. This process depletes cellular NADPH reserves, compromising the regeneration of reduced glutathione and impairing antioxidant defenses. The accumulation of sorbitol within nerve cells creates osmotic stress, leading to cellular swelling and membrane dysfunction.
Simultaneously, the advanced glycation end product (AGE) pathway becomes activated under hyperglycemic conditions. Non-enzymatic glycation of proteins results in the formation of irreversible AGE adducts, which accumulate in nerve tissues and blood vessel walls. AGEs interact with specific receptors, particularly the receptor for AGE (RAGE), triggering inflammatory cascades and oxidative stress responses. The cross-linking properties of AGEs alter protein structure and function, compromising nerve fiber integrity and contributing to the progressive nature of diabetic neuropathy.
The protein kinase C (PKC) pathway activation represents another critical mechanism in diabetic neuropathy pathogenesis. Hyperglycemia increases diacylglycerol synthesis, which activates various PKC isoforms, particularly PKC-β and PKC-δ. Activated PKC promotes vascular permeability, reduces nitric oxide availability, and enhances production of vasoconstrictor factors, ultimately compromising nerve blood flow. PKC activation also influences gene expression patterns, promoting pro-inflammatory and pro-fibrotic responses that contribute to nerve damage progression.
Oxidative Stress and Mitochondrial Dysfunction
Mitochondrial dysfunction emerges as a central theme in diabetic neuropathy pathogenesis, representing both a consequence and perpetuator of hyperglycemic damage. Chronic hyperglycemia overwhelms mitochondrial oxidative capacity, leading to increased production of reactive oxygen species (ROS) and decreased efficiency of ATP synthesis. The electron transport chain becomes dysfunctional, resulting in electron leakage and further ROS generation. This creates a vicious cycle wherein oxidative damage impairs mitochondrial function, which in turn generates more oxidative stress.
The accumulation of oxidative stress overwhelms cellular antioxidant systems, including superoxide dismutase, catalase, and glutathione peroxidase. Lipid peroxidation products, such as 4-hydroxynonenal and malondialdehyde, accumulate in nerve tissues, causing direct damage to membrane phospholipids and proteins. DNA damage occurs through oxidative modification of bases, particularly 8-oxoguanine formation, which can lead to mutations and cellular dysfunction if not properly repaired.
Nitrosative stress compounds the oxidative damage through the formation of peroxynitrite, a potent oxidizing agent formed by the reaction between nitric oxide and superoxide. Peroxynitrite causes protein nitration, lipid peroxidation, and DNA strand breaks, contributing to cellular dysfunction and apoptosis. The interaction between oxidative and nitrosative stress creates a complex web of molecular damage that affects multiple cellular components simultaneously.
Inflammatory Cascades and Neuroinflammation
Neuroinflammation represents a crucial component of diabetic neuropathy pathogenesis, involving both peripheral and central nervous system inflammatory responses. Hyperglycemia activates nuclear factor-κB (NF-κB) signaling pathways, leading to increased expression of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). These cytokines create a pro-inflammatory microenvironment that perpetuates nerve damage and impairs regenerative capacity.
Microglial activation in the dorsal root ganglia and spinal cord represents a key feature of diabetic neuropathy-associated neuroinflammation. Activated microglia release inflammatory mediators, including chemokines, prostaglandins, and matrix metalloproteinases, which contribute to nerve damage and sensitization of pain pathways. The transition from acute to chronic neuroinflammation involves phenotypic changes in microglial cells, shifting from a neuroprotective M2 phenotype to a neurotoxic M1 phenotype.
Complement system activation also contributes to the inflammatory cascade in diabetic neuropathy. AGE-modified proteins and damaged cellular components activate complement pathways, leading to the formation of membrane attack complexes and cellular lysis. Complement activation products, such as C5a, serve as potent chemoattractants for inflammatory cells, amplifying the local inflammatory response.
Vascular and Microcirculatory Alterations
The microvascular hypothesis of diabetic neuropathy emphasizes the role of nerve blood flow alterations in the development and progression of neuropathic complications. Chronic hyperglycemia leads to thickening of capillary basement membranes, endothelial cell dysfunction, and reduced capillary density in nerve tissues. These changes result in compromised oxygen and nutrient delivery to nerve fibers, creating a state of chronic ischemia.
Endothelial dysfunction represents a early and critical event in diabetic microvascular complications. Hyperglycemia impairs endothelial nitric oxide synthase function, reducing nitric oxide availability and compromising vasodilation capacity. Simultaneously, increased production of endothelin-1 and other vasoconstrictor factors promotes vasoconstriction and further reduces nerve blood flow. The imbalance between vasodilatory and vasoconstrictive factors creates a chronic state of impaired microcirculation.
Rheological abnormalities compound the microvascular dysfunction in diabetic neuropathy. Increased blood viscosity, enhanced platelet aggregation, and altered red blood cell deformability contribute to impaired blood flow through small vessels. These rheological changes, combined with structural microvascular alterations, create a complex pathophysiological environment that promotes nerve ischemia and dysfunction.
Ion Channel Dysfunction and Neuronal Hyperexcitability
Diabetic neuropathy involves significant alterations in voltage-gated ion channels, particularly sodium and calcium channels, which contribute to the development of neuropathic pain. Voltage-gated sodium channels, especially Nav1.3, Nav1.7, and Nav1.8 subtypes, become upregulated in damaged sensory neurons, leading to increased membrane excitability and spontaneous firing. This hyperexcitability contributes to the generation of ectopic impulses and the perception of pain in the absence of noxious stimuli.
Calcium channel dysfunction, particularly involving N-type and T-type calcium channels, plays a crucial role in neuropathic pain transmission. Altered calcium channel expression and function affect neurotransmitter release at synaptic terminals and contribute to central sensitization processes. The α2δ subunit of voltage-gated calcium channels becomes a key therapeutic target, as evidenced by the efficacy of gabapentinoids in neuropathic pain management.
Potassium channel dysfunction also contributes to neuronal hyperexcitability in diabetic neuropathy. Downregulation of specific potassium channel subtypes, particularly Kv4.2 and Kv4.3, reduces the ability of neurons to repolarize effectively after action potential generation. This impaired repolarization capacity contributes to prolonged depolarization states and increased firing frequency.
Central Sensitization and Neuroplasticity
Central sensitization represents a fundamental mechanism underlying the chronic pain associated with diabetic neuropathy. Persistent nociceptive input from damaged peripheral nerves leads to functional and structural changes in spinal cord dorsal horn neurons, resulting in amplified pain responses to normal stimuli (allodynia) and enhanced responses to painful stimuli (hyperalgesia). These changes involve alterations in synaptic strength, receptor expression, and intracellular signaling pathways.
NMDA receptor upregulation and enhanced glutamate signaling represent key features of central sensitization in diabetic neuropathy. Increased glutamate release from primary afferent terminals, combined with reduced inhibitory neurotransmission mediated by GABA and glycine, creates an imbalance that favors excitatory transmission. This excitatory-inhibitory imbalance contributes to the maintenance of chronic pain states.
Descending pain modulation systems also become altered in diabetic neuropathy, with dysfunction of both facilitatory and inhibitory pathways. The rostroventromedial medulla, which normally provides balanced descending control over spinal nociceptive transmission, shows altered activity patterns that favor pain facilitation over inhibition. This altered descending control contributes to the persistence and intensity of neuropathic pain.
Clinical Manifestations and Phenotypic Variations
Sensory Manifestations and Pain Characteristics
Diabetic neuropathic pain presents with a diverse array of sensory symptoms that reflect the underlying pathophysiological heterogeneity. The most characteristic feature is the presence of positive symptoms, including spontaneous burning pain, shooting or electric shock-like sensations, and stabbing pain that often follows a stocking-glove distribution. These positive symptoms typically worsen during nighttime hours, contributing to sleep disturbances and significantly impairing quality of life.
Allodynia represents one of the most distressing aspects of diabetic neuropathic pain, characterized by pain perception in response to normally non-painful stimuli such as light touch or clothing contact. This phenomenon reflects the central sensitization processes and altered peripheral nerve function that characterize the condition. Hyperalgesia, manifested as exaggerated pain responses to mildly painful stimuli, often accompanies allodynia and contributes to the overall pain burden.
Negative symptoms, including numbness, reduced vibration sensation, and diminished temperature perception, reflect the progressive loss of nerve fiber function. These symptoms often coexist with positive symptoms, creating a complex clinical picture that can vary significantly between patients. The presence of negative symptoms indicates structural nerve damage and is associated with increased risk of diabetic foot complications.
The temporal pattern of diabetic neuropathic pain shows considerable variation, ranging from intermittent episodes to constant, unrelenting pain. Some patients experience a predominantly nocturnal pattern, while others suffer from continuous symptoms with superimposed acute exacerbations. The unpredictable nature of symptom fluctuations contributes to the psychological burden of the condition and complicates treatment planning.
Motor and Autonomic Involvement
While sensory symptoms predominate in diabetic neuropathy, motor involvement can occur, particularly in advanced cases. Distal muscle weakness typically affects the intrinsic muscles of the feet first, leading to toe deformities, altered gait patterns, and increased risk of falls. Muscle weakness progresses proximally over time, potentially affecting hand function and overall mobility.
Autonomic neuropathy represents a serious complication that can affect multiple organ systems. Cardiovascular autonomic neuropathy manifests as reduced heart rate variability, orthostatic hypotension, and altered blood pressure regulation. Gastrointestinal autonomic involvement can lead to gastroparesis, constipation, or diarrhea, significantly impacting nutritional status and metabolic control.
Sudomotor dysfunction, affecting sweat gland innervation, results in reduced sweating capacity and impaired thermoregulation. This dysfunction contributes to dry skin, increased susceptibility to skin infections, and altered wound healing capacity. The combination of sensory loss and sudomotor dysfunction creates a particularly high risk for diabetic foot complications.
Phenotypic Heterogeneity and Patient Stratification
Recent research has identified distinct phenotypic clusters within diabetic neuropathy populations, reflecting the underlying pathophysiological heterogeneity. Some patients exhibit predominantly inflammatory profiles, characterized by elevated cytokine levels and enhanced immune cell activation. These patients may respond better to anti-inflammatory interventions and immunomodulatory therapies.
Other patients demonstrate primarily metabolic dysfunction patterns, with evidence of severe oxidative stress, mitochondrial impairment, and AGE accumulation. These individuals may benefit more from antioxidant therapies, metabolic modulators, and treatments targeting specific biochemical pathways.
A subset of patients shows mixed phenotypes, combining features of both inflammatory and metabolic dysfunction. These complex cases often require multimodal treatment approaches and present particular therapeutic challenges. Understanding these phenotypic variations is crucial for developing personalized treatment strategies and improving clinical outcomes.
Genetic factors also contribute to phenotypic variability in diabetic neuropathy. Polymorphisms in genes encoding antioxidant enzymes, cytokines, ion channels, and metabolic enzymes can influence disease susceptibility, progression rates, and treatment responses. Pharmacogenomic studies are beginning to identify genetic markers that predict therapeutic efficacy for specific interventions.
Contemporary Treatment Approaches
First-Line Pharmacological Interventions
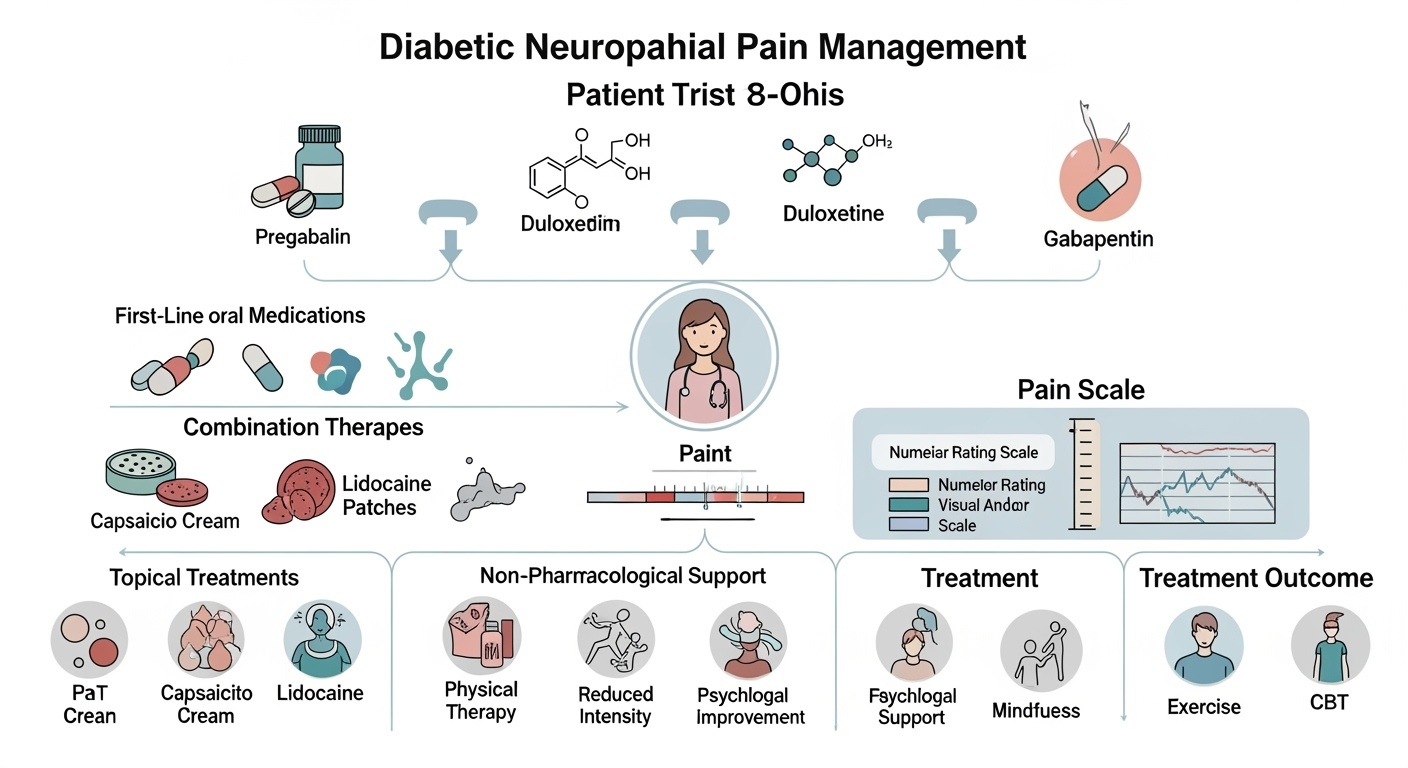
Current evidence-based guidelines recommend specific pharmacological agents as first-line treatments for diabetic neuropathic pain, based on their demonstrated efficacy and acceptable safety profiles. Pregabalin, an α2δ calcium channel ligand, represents one of the most extensively studied and widely prescribed medications for this indication. Its mechanism involves binding to the α2δ subunit of voltage-gated calcium channels, reducing calcium influx and subsequent neurotransmitter release. Clinical trials have consistently demonstrated pregabalin’s efficacy in reducing pain intensity and improving sleep quality, with benefits typically emerging within the first week of treatment.
Duloxetine, a balanced serotonin-norepinephrine reuptake inhibitor, offers another first-line option with a dual mechanism of action. Beyond its effects on monoamine neurotransmitter systems, duloxetine modulates descending pain inhibitory pathways and may have direct effects on sodium channel function. The medication demonstrates particular efficacy in patients with comorbid depression or anxiety, addressing both pain and mood symptoms simultaneously. Clinical studies have shown sustained efficacy over extended treatment periods, with benefits maintained for up to one year of continuous therapy.
Gabapentin, while sharing a similar mechanism of action with pregabalin, shows somewhat different pharmacokinetic properties and side effect profiles. Its absorption is saturable and dose-dependent, requiring more frequent dosing to achieve therapeutic levels. Despite these limitations, gabapentin remains an effective option, particularly for patients who do not tolerate pregabalin or when cost considerations are paramount. The medication’s long clinical track record and extensive safety database make it a reliable choice for many patients.
Tricyclic Antidepressants and Alternative Antidepressants
Tricyclic antidepressants, particularly amitriptyline and nortriptyline, have demonstrated significant efficacy in diabetic neuropathic pain management through multiple mechanisms of action. These medications block sodium channels, inhibit norepinephrine and serotonin reuptake, and possess anticholinergic and antihistaminergic properties that contribute to their analgesic effects. However, their use is often limited by anticholinergic side effects, including dry mouth, constipation, urinary retention, and cognitive impairment, particularly in elderly patients.
Nortriptyline offers a more favorable side effect profile compared to amitriptyline, with reduced anticholinergic burden while maintaining comparable analgesic efficacy. The medication’s secondary amine structure results in less pronounced anticholinergic effects, making it a preferred choice when tricyclic therapy is indicated. Careful dose titration and monitoring for cardiovascular effects are essential, particularly in patients with preexisting cardiac conditions.
Venlafaxine, another serotonin-norepinephrine reuptake inhibitor, provides an alternative to duloxetine with similar efficacy but different pharmacokinetic properties. The medication’s shorter half-life requires more frequent dosing but may offer advantages in terms of discontinuation symptoms and drug interactions. Extended-release formulations improve dosing convenience and compliance while maintaining therapeutic efficacy.
Topical Therapies and Localized Treatments
Topical treatments offer advantages for patients who experience systemic side effects from oral medications or prefer localized therapy. Capsaicin, derived from chili peppers, acts through initial activation followed by desensitization of TRPV1 receptors on nociceptive nerve terminals. High-concentration capsaicin patches (8%) provide prolonged pain relief through local depletion of substance P and other neuropeptides, with effects lasting several months after a single application.
Lidocaine patches represent another topical option, providing local anesthetic effects through sodium channel blockade. The 5% lidocaine patch demonstrates efficacy in localized neuropathic pain, with minimal systemic absorption and few side effects. The patches can be applied directly to painful areas and provide flexible dosing options based on individual patient needs and preferences.
Compounded topical preparations combining multiple active ingredients offer customized treatment options for complex cases. Combinations may include local anesthetics, anti-inflammatory agents, antidepressants, and anticonvulsants in formulations tailored to individual patient requirements. While evidence for these combinations remains limited, clinical experience suggests potential benefits in selected patients.
Emerging Pharmacological Approaches
Novel sodium channel blockers represent an exciting area of development in neuropathic pain treatment. Selective Nav1.7 and Nav1.8 channel inhibitors aim to reduce pathological nerve firing while preserving normal neuronal function. These agents promise improved efficacy with reduced side effects compared to current medications, though clinical development has proven challenging due to the complexity of sodium channel pharmacology.
Nerve growth factor (NGF) antagonists offer a unique approach by targeting the underlying mechanisms of nerve sensitization. Monoclonal antibodies against NGF, such as tanezumab, have shown promising results in clinical trials for various chronic pain conditions. However, concerns about potential joint safety issues have slowed development, requiring careful risk-benefit evaluation.
Cannabinoid-based therapies are gaining attention as potential treatments for neuropathic pain. Both plant-derived and synthetic cannabinoids demonstrate analgesic properties through interaction with CB1 and CB2 receptors. Clinical evidence remains mixed, with some studies showing modest benefits while others demonstrate limited efficacy. The legal and regulatory landscape surrounding cannabinoid medications continues to evolve.
Advanced Therapeutic Strategies and Combination Approaches
Multimodal Combination Therapy
The complex and multifactorial nature of diabetic neuropathic pain often necessitates combination therapy approaches that target multiple pathophysiological mechanisms simultaneously. Evidence-based combination strategies have emerged from systematic clinical investigations, demonstrating superior efficacy compared to monotherapy in many patients. The combination of pregabalin with duloxetine represents one of the most extensively studied approaches, with clinical trials showing additive analgesic effects and improved functional outcomes.
Mechanistic rationale supports the combination of medications with different modes of action, as this approach can address the diverse pathophysiological processes underlying neuropathic pain. Combining calcium channel modulators with monoamine reuptake inhibitors provides both peripheral nerve stabilization and central pain modulation, potentially achieving more comprehensive pain control than either approach alone. The COMBO-DN study demonstrated that pregabalin-duloxetine combination therapy achieved superior pain reduction compared to either medication used as monotherapy.
Alpha-lipoic acid represents a valuable addition to combination regimens, particularly when combined with conventional neuropathic pain medications. The antioxidant properties of alpha-lipoic acid address the oxidative stress component of diabetic neuropathy pathogenesis, while its metabolic effects may provide disease-modifying benefits. Clinical studies have shown that alpha-lipoic acid combined with pregabalin or gabapentin produces enhanced pain relief and improved nerve conduction parameters compared to anticonvulsant monotherapy.
The combination of oral medications with topical therapies offers another strategic approach, allowing for systemic pain modulation combined with localized treatment. Patients who achieve partial response to oral therapy may benefit from the addition of capsaicin patches or lidocaine preparations, providing additional pain relief while potentially reducing systemic medication requirements. This approach is particularly valuable for patients experiencing dose-limiting side effects from oral medications.
Interventional Pain Management Techniques
Spinal cord stimulation has emerged as a valuable treatment option for refractory diabetic neuropathic pain that fails to respond adequately to pharmacological interventions. High-frequency (10 kHz) spinal cord stimulation demonstrates particular promise, with clinical studies showing sustained pain relief and improved quality of life measures. The mechanism involves modulation of spinal cord pain processing circuits, reducing both spontaneous pain and evoked pain responses.
The SENZA-PDN study provided robust evidence for high-frequency spinal cord stimulation efficacy in painful diabetic neuropathy, with 79% of patients achieving clinically meaningful pain reduction at 12 months. The therapy demonstrates durability of effect, with sustained benefits observed over extended follow-up periods. Patient selection criteria include adequate trial period with conservative management, confirmed diagnosis of diabetic neuropathy, and psychological evaluation to ensure optimal outcomes.
Peripheral nerve stimulation represents an alternative interventional approach for localized neuropathic pain. This technique involves implantation of stimulation leads near affected peripheral nerves, providing targeted treatment for specific anatomical regions. Peripheral nerve stimulation may be particularly suitable for patients with focal neuropathic pain distributions or those who are not candidates for spinal cord stimulation.
Sympathetic nerve blocks and neurolytic procedures may provide benefit in selected patients with diabetic neuropathy, particularly those with significant autonomic involvement or complex regional pain syndrome features. These procedures require careful patient selection and should be performed by experienced interventional pain specialists. The evidence base for these techniques in diabetic neuropathy remains limited, requiring individualized risk-benefit assessment.
Regenerative and Neuroprotective Approaches
Mesenchymal stem cell therapy represents a promising regenerative approach for diabetic neuropathy, with preclinical studies demonstrating nerve regeneration and functional recovery. Clinical trials are investigating various stem cell sources, including bone marrow-derived, adipose-derived, and umbilical cord-derived mesenchymal stem cells. The proposed mechanisms include paracrine factor secretion, direct cell replacement, and immunomodulatory effects that promote nerve repair and regeneration.
Growth factor therapies target the underlying nerve damage in diabetic neuropathy through promotion of axonal regeneration and Schwann cell proliferation. Nerve growth factor, brain-derived neurotrophic factor, and insulin-like growth factor-1 have shown promise in preclinical studies. Clinical translation has proven challenging due to issues with drug delivery, dosing optimization, and potential side effects. Novel delivery systems, including nanoparticle formulations and gene therapy vectors, are being developed to overcome these limitations.
Exosome-based therapies represent an innovative approach that harnesses the regenerative potential of stem cell-derived extracellular vesicles. Exosomes contain growth factors, microRNAs, and other bioactive molecules that can promote nerve regeneration and reduce inflammation. This approach offers potential advantages over cell-based therapies, including improved safety profile, easier storage and handling, and reduced immunogenicity.
Gene therapy approaches are being investigated for diabetic neuropathy treatment, with strategies including gene delivery for growth factor expression, antioxidant enzyme upregulation, and pro-survival factor enhancement. Viral vectors and non-viral delivery systems are being optimized for peripheral nerve targeting. The VM202 clinical program, involving hepatocyte growth factor gene therapy, demonstrated promising results in early-phase trials, though subsequent larger studies showed mixed outcomes.
Novel Therapeutic Targets and Future Directions
Emerging Molecular Targets
The expanding understanding of diabetic neuropathy pathophysiology has revealed numerous novel therapeutic targets that offer promise for more effective treatments. Autophagy modulation represents one such target, as impaired autophagy contributes to the accumulation of damaged cellular components and mitochondrial dysfunction in diabetic neuropathy. Autophagy enhancers, such as rapamycin analogs and trehalose, are being investigated for their potential to restore cellular homeostasis and promote nerve repair.
The endoplasmic reticulum stress response pathway has emerged as another important target, as chronic hyperglycemia induces endoplasmic reticulum stress and activates the unfolded protein response. This cellular stress response contributes to neuronal dysfunction and death in diabetic neuropathy. Chemical chaperones and endoplasmic reticulum stress modulators are being developed to reduce cellular stress and improve neuronal survival.
Epigenetic modifications, including DNA methylation and histone modifications, play important roles in diabetic neuropathy pathogenesis. Histone deacetylase inhibitors have shown neuroprotective effects in preclinical studies and are being investigated in clinical trials. The ability to modulate gene expression through epigenetic mechanisms offers opportunities for disease modification rather than purely symptomatic treatment.
The gut-brain-peripheral nerve axis represents a newly recognized pathway in diabetic neuropathy pathogenesis. Alterations in gut microbiota composition and function contribute to systemic inflammation and metabolic dysfunction that affect peripheral nerve health. Microbiome modulation through probiotics, prebiotics, and fecal microbiota transplantation is being investigated as a potential therapeutic approach.
Precision Medicine and Personalized Treatment
Pharmacogenomic approaches are beginning to inform personalized treatment decisions in diabetic neuropathy management. Genetic variations in drug-metabolizing enzymes, transporters, and targets can significantly influence treatment response and side effect susceptibility. CYP2D6 polymorphisms affect tricyclic antidepressant metabolism, while variations in calcium channel subunit genes may influence gabapentinoid efficacy.
Biomarker-guided therapy represents another aspect of precision medicine in diabetic neuropathy. Inflammatory biomarkers, oxidative stress markers, and nerve-specific proteins can provide insights into individual pathophysiological profiles and guide treatment selection. Patients with elevated inflammatory markers may benefit more from anti-inflammatory interventions, while those with prominent oxidative stress may respond better to antioxidant therapies.
Machine learning and artificial intelligence approaches are being applied to identify patient subgroups and predict treatment responses. These technologies can integrate multiple data sources, including clinical characteristics, biomarkers, genetic information, and imaging data, to develop personalized treatment algorithms. Early applications have shown promise in identifying patients most likely to respond to specific interventions.
Wearable technology and remote monitoring systems offer opportunities for personalized treatment optimization through continuous assessment of symptoms, activity levels, and treatment responses. These technologies can provide real-time feedback to both patients and healthcare providers, enabling more responsive treatment adjustments and improved outcomes.
Integration of Digital Health Technologies
Digital therapeutics represent an emerging category of evidence-based interventions delivered through software platforms to prevent, manage, or treat medical conditions. For diabetic neuropathy, digital therapeutics applications may include pain self-management tools, medication adherence support, and behavioral interventions for sleep and mood management. These platforms can provide personalized content based on individual patient characteristics and treatment responses.
Virtual reality and augmented reality technologies are being explored as novel pain management tools for diabetic neuropathy. These immersive technologies can provide distraction therapy, relaxation training, and graded exposure interventions that may help reduce pain perception and improve coping strategies. Early studies suggest potential benefits, though more research is needed to establish efficacy and optimal implementation strategies.
Telemedicine and remote care models have become increasingly important for diabetic neuropathy management, particularly in underserved populations with limited access to specialized care. These approaches can provide expert consultation, medication management, and ongoing monitoring while reducing travel burden and healthcare costs. Integration with remote monitoring technologies can enhance care quality and patient outcomes.
Comparative Efficacy Analysis
| Treatment Category | Mechanism of Action | Primary Efficacy Outcomes | Notable Side Effects | Evidence Quality |
| Pregabalin | α2δ calcium channel ligand | 30-50% pain reduction in 40-60% of patients | Dizziness, weight gain, peripheral edema | High (multiple RCTs) |
| Duloxetine | SNRI antidepressant | 30-50% pain reduction in 50-70% of patients | Nausea, somnolence, constipation | High (multiple RCTs) |
| Gabapentin | α2δ calcium channel ligand | 30-50% pain reduction in 35-45% of patients | Dizziness, somnolence, peripheral edema | Moderate (fewer RCTs) |
| Tricyclic Antidepressants | Multi-target (Na+ channels, monoamine reuptake) | 30-50% pain reduction in 40-50% of patients | Anticholinergic effects, cardiac risks | Moderate (older studies) |
| Alpha-lipoic Acid | Antioxidant, metabolic modulator | Modest pain reduction, improved nerve conduction | Minimal (GI upset, skin reactions) | Moderate (mixed results) |
| Topical Capsaicin | TRPV1 receptor agonist/desensitizer | Localized pain reduction in 30-40% of patients | Local burning, erythema | Moderate (limited studies) |
| Advanced Therapies | Mechanism of Action | Clinical Evidence | Current Status | Future Potential |
| High-frequency SCS | Spinal cord modulation | 70-80% responder rate in selected patients | Established interventional option | Expanding indications |
| Combination Therapy | Multi-modal approach | Enhanced efficacy vs. monotherapy | Increasing clinical adoption | Personalized combinations |
| Stem Cell Therapy | Regenerative mechanisms | Promising preclinical, early clinical data | Investigational | High regenerative potential |
| Gene Therapy | Growth factor delivery | Mixed clinical trial results | Experimental | Disease-modifying potential |
| Novel Na+ Channel Blockers | Selective channel inhibition | Early clinical development | Investigational | Improved selectivity profile |
Conclusions and Clinical Implications
Diabetic neuropathy represents a complex, multifaceted condition that requires comprehensive understanding of its diverse pathophysiological mechanisms to optimize therapeutic approaches. The evolution from purely symptom-based treatment to mechanism-targeted interventions reflects our growing appreciation of the condition’s complexity and the need for personalized management strategies. Current evidence supports a multimodal approach that combines pharmacological interventions with non-pharmacological strategies, tailored to individual patient characteristics and pathophysiological profiles.
The emergence of precision medicine approaches holds particular promise for improving outcomes in diabetic neuropathy management. By integrating genetic, biomarker, and clinical data, healthcare providers can better predict treatment responses and optimize therapeutic regimens for individual patients. This personalized approach has the potential to improve efficacy while minimizing adverse effects, addressing one of the major limitations of current treatment paradigms.
Future research priorities should focus on developing disease-modifying therapies that can halt or reverse the progression of diabetic neuropathy, rather than merely managing symptoms. Regenerative approaches, including stem cell therapies and growth factor treatments, offer promise for nerve repair and functional recovery. Additionally, the integration of digital health technologies and artificial intelligence may revolutionize how we monitor, diagnose, and treat diabetic neuropathy, providing more responsive and effective care.
The successful management of diabetic neuropathy requires a multidisciplinary approach involving endocrinologists, neurologists, pain specialists, and primary care providers working together to address the diverse aspects of this complex condition. Patient education and self-management support remain crucial components of comprehensive care, empowering individuals to actively participate in their treatment and optimize their outcomes. As our understanding of diabetic neuropathy continues to evolve, the integration of new scientific insights with clinical expertise will drive continued improvements in patient care and quality of life.
 glucosesafe.com
glucosesafe.com