Advanced Diabetes Technology: Artificial Pancreas Systems, Closed-Loop Control, and the Future of Automated Insulin Delivery
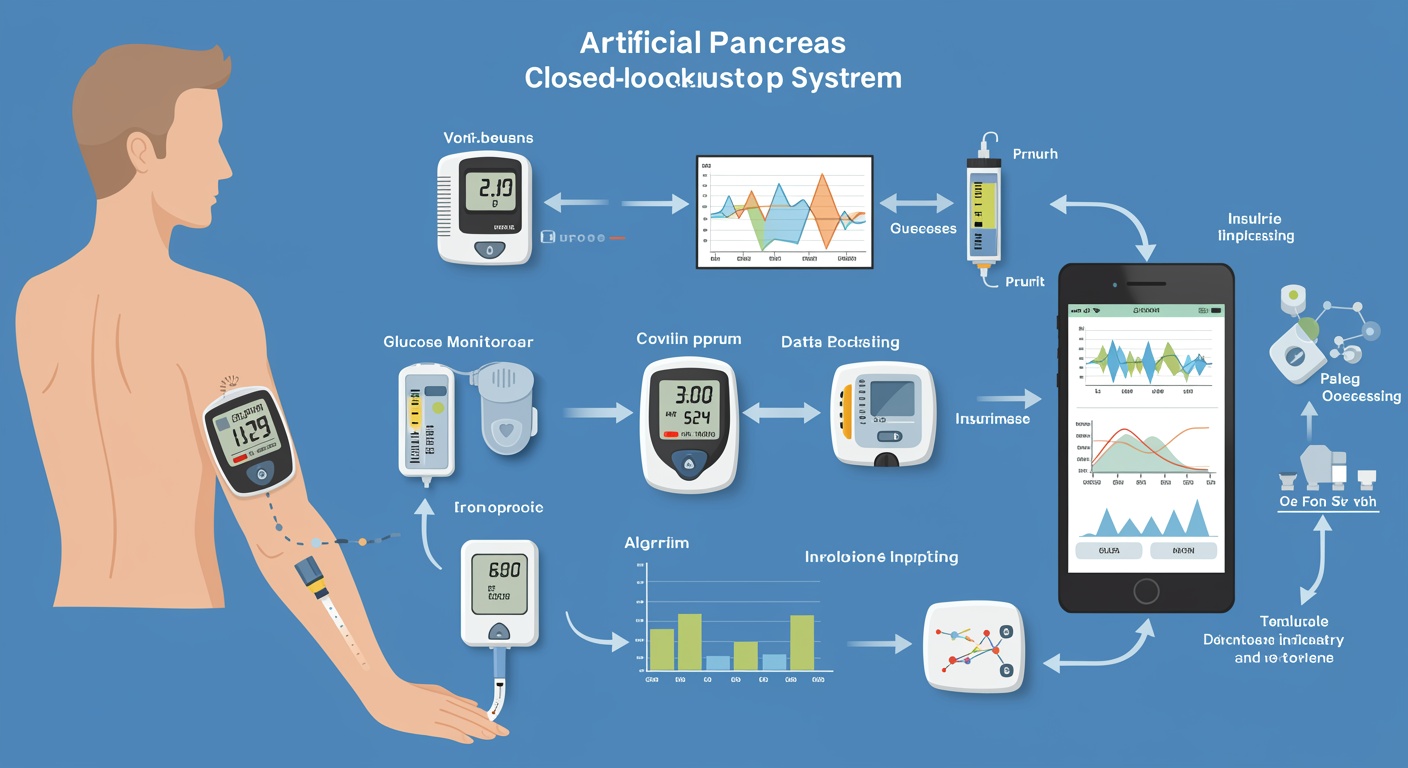
The management of diabetes mellitus has undergone a revolutionary transformation over the past decade, driven by remarkable advances in biomedical engineering, sensor technology, and computational algorithms that have culminated in the development of sophisticated artificial pancreas systems. These innovative therapeutic platforms represent the convergence of continuous glucose monitoring technology, automated insulin delivery systems, and intelligent control algorithms designed to mimic the physiological function of pancreatic beta cells in maintaining glucose homeostasis. The evolution from manual insulin administration to fully automated closed-loop systems marks a paradigm shift that promises to fundamentally alter the landscape of diabetes care and significantly improve the quality of life for millions of individuals living with type 1 diabetes and insulin-requiring type 2 diabetes.
The concept of an artificial pancreas has captured the imagination of researchers, clinicians, and patients for decades, representing the ultimate goal of diabetes technology development. Unlike traditional diabetes management approaches that rely heavily on patient self-management and frequent manual interventions, artificial pancreas systems offer the promise of continuous, automated glucose control with minimal user intervention. These systems integrate real-time glucose sensing with predictive algorithms and automated insulin delivery to provide personalized, adaptive diabetes management that responds dynamically to changing physiological conditions, lifestyle factors, and individual patient characteristics.
The clinical significance of artificial pancreas technology extends far beyond simple glucose control, encompassing profound implications for reducing the burden of diabetes self-management, preventing acute and chronic complications, and improving psychological well-being. The constant vigilance required for optimal diabetes management often leads to diabetes distress, sleep disruption, and impaired quality of life, challenges that automated systems are uniquely positioned to address. By reducing the cognitive and emotional burden of diabetes management while simultaneously improving glycemic outcomes, artificial pancreas systems represent a transformative therapeutic approach that addresses both the physiological and psychosocial aspects of living with diabetes.
Historical Evolution and Technological Foundations
The journey toward artificial pancreas systems began in the 1970s with pioneering work in glucose sensing and feedback control, building upon fundamental principles of biomedical engineering and control theory. Early efforts focused on developing reliable glucose sensors capable of providing continuous, accurate measurements of blood glucose concentrations, a prerequisite for any automated insulin delivery system. The initial glucose sensors were plagued by accuracy limitations, calibration requirements, and biocompatibility issues that significantly hindered clinical application. Parallel developments in insulin delivery technology, including the refinement of continuous subcutaneous insulin infusion pumps, provided the foundation for automated insulin administration systems.
The evolution of continuous glucose monitoring technology has been instrumental in enabling artificial pancreas development, with successive generations of sensors demonstrating improved accuracy, reduced calibration requirements, and enhanced user experience. Modern continuous glucose monitoring systems utilize advanced electrochemical sensing principles, sophisticated calibration algorithms, and improved biocompatible materials to provide real-time glucose measurements with accuracy approaching that of traditional blood glucose meters. The integration of wireless communication capabilities and mobile connectivity has further enhanced the utility of these sensors, enabling seamless data transmission to insulin pumps and smartphone applications.
Insulin pump technology has undergone concurrent evolution, with modern devices offering precise insulin delivery, programmable delivery profiles, and sophisticated safety features designed to prevent insulin overdosing and device malfunctions. The miniaturization of pump components, improvements in battery technology, and the development of rapid-acting insulin formulations have collectively enhanced the feasibility and effectiveness of continuous subcutaneous insulin infusion. The integration of wireless communication protocols has enabled pumps to receive real-time glucose data and algorithm-generated insulin delivery commands, forming the foundation for closed-loop control systems.
The development of control algorithms represents the intellectual heart of artificial pancreas systems, requiring sophisticated mathematical models that can predict glucose dynamics, optimize insulin delivery, and adapt to individual patient characteristics. Early control algorithms were based on proportional-integral-derivative controllers adapted from industrial automation, but the unique challenges of glucose regulation, including significant time delays, individual variability, and meal-related disturbances, necessitated the development of more sophisticated approaches. Advanced control strategies, including model predictive control, fuzzy logic controllers, and machine learning algorithms, have emerged as promising approaches for managing the complex dynamics of glucose regulation.
Physiological Principles and System Requirements
Understanding the physiological principles underlying normal glucose homeostasis is fundamental to appreciating the challenges and opportunities in artificial pancreas development. The healthy pancreas maintains glucose concentrations within a narrow range through the coordinated actions of insulin-secreting beta cells and glucagon-secreting alpha cells, responding to changes in glucose levels with remarkable precision and speed. Beta cells exhibit a biphasic insulin secretion pattern, with rapid first-phase insulin release followed by sustained second-phase secretion, providing both immediate glucose control and long-term metabolic regulation. This sophisticated physiological control system must be replicated, to the extent possible, by artificial pancreas systems.
The pharmacokinetics and pharmacodynamics of subcutaneous insulin delivery present significant challenges for artificial pancreas systems, as the absorption and action profiles of subcutaneous insulin differ substantially from physiological insulin secretion. Subcutaneous insulin absorption is influenced by factors including injection site characteristics, blood flow, insulin concentration, and individual patient factors, creating variability that must be accounted for in control algorithms. The time delay between insulin administration and glucose-lowering effect, typically ranging from thirty to ninety minutes, requires predictive control strategies that can anticipate glucose changes and adjust insulin delivery proactively.
Meal-related glucose excursions represent one of the most challenging aspects of artificial pancreas control, as food intake introduces large, unpredictable disturbances to the glucose system that must be managed effectively to prevent hyperglycemia. The timing, composition, and quantity of meals significantly influence postprandial glucose patterns, requiring control algorithms that can adapt to diverse dietary patterns and individual metabolic responses. Exercise and physical activity present additional challenges, as increased glucose utilization and altered insulin sensitivity during and after exercise can lead to hypoglycemia if not properly managed by the control system.
Individual variability in insulin sensitivity, glucose production, and absorption rates necessitates personalized control algorithms that can adapt to patient-specific characteristics and changing physiological conditions. Factors such as stress, illness, hormonal fluctuations, and medication use can significantly alter glucose dynamics, requiring control systems that can detect and respond to these changes. The development of adaptive algorithms that can learn from individual glucose patterns and adjust control parameters accordingly represents a major focus of current research efforts.
Control Algorithm Design and Implementation
The design of effective control algorithms for artificial pancreas systems requires sophisticated mathematical modeling of glucose-insulin dynamics combined with robust control strategies that can manage uncertainty, disturbances, and system constraints. Model predictive control has emerged as a leading approach for artificial pancreas applications, offering the ability to predict future glucose concentrations based on current measurements and planned insulin delivery while optimizing control actions over a prediction horizon. These algorithms incorporate mathematical models of glucose-insulin dynamics, constraints on insulin delivery rates, and safety limits designed to prevent hypoglycemia and excessive hyperglycemia.
The development of accurate glucose-insulin models is crucial for effective predictive control, requiring the integration of physiological knowledge with patient-specific parameter estimation. These models must capture the essential dynamics of glucose absorption, insulin action, and glucose production while remaining computationally tractable for real-time implementation. Compartmental models based on physiological principles provide a framework for understanding glucose-insulin interactions, while data-driven approaches using machine learning techniques offer the potential for improved model accuracy and personalization.
Safety considerations are paramount in artificial pancreas algorithm design, as system failures or inappropriate insulin delivery can result in severe hypoglycemia or diabetic ketoacidosis. Multiple layers of safety protection are typically implemented, including insulin delivery limits, glucose trend monitoring, and fail-safe mechanisms that suspend insulin delivery when glucose levels approach hypoglycemic ranges. Predictive low glucose suspend algorithms represent an important intermediate step in artificial pancreas development, automatically suspending insulin delivery when hypoglycemia is predicted based on glucose trends and algorithm projections.
The incorporation of meal and exercise information into control algorithms represents an area of active research, with approaches ranging from manual meal announcements to automated meal detection systems. Meal announcement strategies require users to input information about carbohydrate intake, allowing the algorithm to provide appropriate prandial insulin delivery. Automated meal detection algorithms attempt to identify meals based on glucose sensor data patterns, potentially reducing the burden of meal announcement while maintaining effective postprandial glucose control.
Current Commercial Systems and Clinical Applications
The commercial landscape of artificial pancreas systems has evolved rapidly, with several FDA-approved hybrid closed-loop systems now available for clinical use. The Medtronic MiniMed 780G system represents a significant advancement in automated insulin delivery, featuring advanced hybrid closed-loop technology that automatically adjusts basal insulin delivery and provides correction boluses based on continuous glucose monitoring data. The system incorporates sophisticated control algorithms that can adapt to individual insulin requirements and provide personalized glucose control with minimal user intervention beyond meal bolus administration.
The Tandem t:slim X2 insulin pump with Control-IQ technology offers another commercial hybrid closed-loop solution, utilizing predictive algorithms to automatically adjust insulin delivery and prevent both hypoglycemia and hyperglycemia. The Control-IQ algorithm incorporates sleep and exercise activity modes that modify control parameters to optimize glucose management during different daily activities. The system demonstrates the practical implementation of advanced control strategies in a user-friendly platform that integrates seamlessly with continuous glucose monitoring systems.
The Omnipod 5 system represents the first tubeless, wearable hybrid closed-loop system, offering enhanced convenience and discretion for users while maintaining sophisticated automated insulin delivery capabilities. The system utilizes adaptive control algorithms that learn from individual glucose patterns and adjust control parameters to optimize personalized glucose management. The integration with smartphone applications provides users with comprehensive diabetes management tools while maintaining the automated insulin delivery functionality.
Clinical outcomes from real-world use of commercial artificial pancreas systems have demonstrated significant improvements in glycemic control, time in range, and user satisfaction compared to traditional insulin delivery methods. Studies have consistently shown increased time in target glucose range, reduced hypoglycemia, and improved hemoglobin A1c levels with hybrid closed-loop systems. The reduction in diabetes management burden has been associated with improved quality of life measures and reduced diabetes distress scores, highlighting the psychosocial benefits of automated insulin delivery technology.
Advanced Control Strategies and Machine Learning Applications
The integration of machine learning and artificial intelligence techniques into artificial pancreas control represents a frontier area with tremendous potential for improving system performance and personalization. Machine learning algorithms can analyze vast amounts of glucose, insulin, and lifestyle data to identify patterns and relationships that may not be apparent through traditional modeling approaches. These techniques offer the potential for more accurate glucose prediction, personalized control parameter optimization, and adaptive algorithm behavior that improves over time with accumulated experience.
Deep learning neural networks have shown promise for glucose prediction applications, with convolutional and recurrent neural network architectures demonstrating superior prediction accuracy compared to traditional mathematical models in some studies. These approaches can potentially capture complex, nonlinear relationships in glucose dynamics while adapting to individual patient characteristics without requiring explicit physiological model specification. The challenge lies in ensuring robustness, interpretability, and safety when implementing these approaches in clinical artificial pancreas systems.
Reinforcement learning represents another promising machine learning approach for artificial pancreas control, offering the potential for algorithms that can learn optimal control policies through interaction with the glucose control environment. These approaches can potentially adapt to changing patient characteristics, optimize long-term glucose control objectives, and balance competing goals such as tight glucose control versus hypoglycemia avoidance. The development of safe and effective reinforcement learning algorithms for artificial pancreas applications remains an active area of research.
Federated learning approaches offer the potential for artificial pancreas systems to benefit from collective learning across patient populations while maintaining individual privacy and data security. These techniques enable algorithms to learn from diverse patient experiences and improve performance for all users without requiring centralized data sharing. The application of federated learning to artificial pancreas systems could potentially accelerate algorithm improvement and enable personalization based on population-level insights.
Sensor Technology and Integration Challenges
The accuracy and reliability of continuous glucose monitoring systems remain critical factors limiting the performance of artificial pancreas systems, as control algorithms depend entirely on glucose sensor data for decision-making. Current continuous glucose monitoring systems measure glucose concentrations in interstitial fluid rather than blood, introducing physiological lag times and potential accuracy limitations that must be accounted for in control algorithm design. The development of more accurate, stable, and responsive glucose sensors represents a key area for continued technological advancement.
Sensor calibration requirements and accuracy drift over time present ongoing challenges for artificial pancreas applications, as control algorithms must maintain effective performance despite potential sensor inaccuracies. Advanced calibration algorithms and sensor fusion techniques that combine multiple sensor inputs may offer solutions for improving overall system accuracy and reliability. The development of calibration-free sensors represents an important goal for reducing user burden and improving system convenience.
The integration of additional physiological sensors beyond glucose monitoring offers the potential for enhanced artificial pancreas performance through more comprehensive metabolic monitoring. Heart rate monitoring, accelerometry, and other physiological measurements can provide valuable information about physical activity, stress, and metabolic state that could be incorporated into control algorithms. The challenge lies in developing robust sensor fusion algorithms that can effectively utilize multiple data streams while maintaining system simplicity and reliability.
Communication and connectivity challenges between system components require robust wireless protocols and fail-safe mechanisms to ensure reliable operation. The artificial pancreas system must maintain effective communication between the glucose sensor, insulin pump, and control algorithm while managing potential interference, signal loss, and device failures. The development of redundant communication pathways and graceful degradation modes ensures system safety and reliability under adverse conditions.
Safety Mechanisms and Risk Management
The implementation of comprehensive safety mechanisms is paramount in artificial pancreas system design, as the automated nature of insulin delivery creates unique safety challenges that must be addressed through multiple layers of protection. Primary safety mechanisms focus on preventing severe hypoglycemia through predictive algorithms that can anticipate glucose decreases and suspend or reduce insulin delivery before dangerous glucose levels are reached. These predictive low glucose suspend features represent a critical safety component that has demonstrated effectiveness in reducing hypoglycemic episodes in clinical studies.
Secondary safety mechanisms include maximum insulin delivery limits, glucose rate-of-change monitoring, and fail-safe modes that activate when sensor accuracy is compromised or communication failures occur. These systems must balance aggressive glucose control with hypoglycemia prevention, requiring sophisticated algorithms that can assess risk and adjust control parameters accordingly. The incorporation of user-adjustable safety parameters allows for personalization of risk management strategies based on individual preferences and clinical circumstances.
System redundancy and failure mode management represent critical aspects of artificial pancreas safety design, ensuring that device failures do not result in dangerous insulin delivery or system shutdown. Backup communication pathways, redundant safety checks, and graceful degradation modes help maintain system functionality even when individual components fail. The development of comprehensive system monitoring and alert mechanisms enables users and healthcare providers to detect and respond to potential safety issues before they become clinically significant.
Post-market surveillance and real-world safety data collection are essential for continued artificial pancreas system improvement and risk management. The accumulation of large-scale safety and performance data from commercial systems provides valuable insights into rare adverse events, system limitations, and opportunities for improvement. The integration of remote monitoring capabilities enables healthcare providers to track system performance and intervene when safety concerns arise.
Clinical Outcomes and Real-World Performance
Clinical studies of artificial pancreas systems have consistently demonstrated significant improvements in glycemic control metrics compared to traditional diabetes management approaches, with particular benefits observed in time in target glucose range and reduction of hypoglycemic episodes. Randomized controlled trials have shown that hybrid closed-loop systems can achieve time in range values of seventy to eighty percent, representing substantial improvements over conventional insulin delivery methods. These improvements are accompanied by reductions in glycemic variability and more stable overnight glucose control.
The reduction in severe hypoglycemia represents one of the most clinically significant benefits of artificial pancreas systems, as these episodes can result in emergency department visits, hospitalizations, and potentially life-threatening complications. Clinical studies have demonstrated significant reductions in both biochemical and severe hypoglycemia with closed-loop systems, providing important safety benefits for users. The predictive algorithms and automated insulin suspension capabilities of these systems are particularly effective at preventing nocturnal hypoglycemia, a major concern for individuals with type 1 diabetes.
Real-world evidence from commercial artificial pancreas systems has generally confirmed the benefits observed in clinical trials, with some studies suggesting even greater improvements in diabetes outcomes when systems are used in routine clinical practice. The integration of artificial pancreas technology into real-world diabetes care has demonstrated feasibility across diverse patient populations and healthcare settings. User satisfaction and quality of life measures consistently show improvements with artificial pancreas systems, reflecting both the clinical benefits and reduced management burden associated with automated insulin delivery.
Long-term clinical outcomes and complications prevention represent important areas for continued research, as the ultimate goal of improved diabetes technology is to reduce the risk of chronic complications such as cardiovascular disease, nephropathy, retinopathy, and neuropathy. While short-term studies have demonstrated improved glycemic control, longer-term studies are needed to definitively establish the impact of artificial pancreas systems on diabetes complications and overall health outcomes.
Patient Selection and Clinical Implementation
The successful implementation of artificial pancreas systems in clinical practice requires careful patient selection, comprehensive education, and ongoing clinical support to optimize system performance and user experience. Ideal candidates for artificial pancreas therapy typically include individuals with type 1 diabetes or insulin-requiring type 2 diabetes who have experience with insulin pump therapy and continuous glucose monitoring. Previous experience with diabetes technology helps ensure that users have the technical skills and motivation necessary for successful artificial pancreas system use.
Patient education and training programs are critical for successful artificial pancreas implementation, as users must understand system operation, safety features, and troubleshooting procedures to maximize benefits and minimize risks. Comprehensive training should address carbohydrate counting, meal bolus administration, exercise management, and system maintenance procedures. The development of standardized training curricula and certification programs helps ensure consistent, high-quality education across different healthcare settings.
Healthcare provider education and support systems are equally important for successful artificial pancreas implementation, as clinicians must understand system capabilities, limitations, and optimization strategies to provide effective clinical care. The complexity of artificial pancreas systems requires specialized knowledge that extends beyond traditional diabetes management, encompassing device troubleshooting, algorithm optimization, and data interpretation skills. The development of clinical decision support tools and professional education programs helps prepare healthcare providers for artificial pancreas management.
Ongoing clinical support and system optimization represent important aspects of artificial pancreas care, as these systems require periodic adjustments and monitoring to maintain optimal performance. Remote monitoring capabilities and telemedicine platforms can facilitate ongoing care while reducing the burden of frequent clinic visits. The integration of artificial pancreas data with electronic health records and clinical decision support systems enables more comprehensive and coordinated diabetes care.
Economic Considerations and Healthcare System Integration
The economic impact of artificial pancreas systems encompasses both the substantial upfront costs of these sophisticated medical devices and the potential long-term healthcare savings associated with improved diabetes control and complications prevention. The high initial cost of artificial pancreas systems, including continuous glucose monitoring sensors, insulin pumps, and control algorithms, presents a significant barrier to access for many patients and healthcare systems. Insurance coverage and reimbursement policies vary widely, creating disparities in access to these technologies.
Cost-effectiveness analyses of artificial pancreas systems must consider both direct medical costs and indirect costs associated with hypoglycemic episodes, emergency department visits, hospitalizations, and long-term complications. While the upfront costs are substantial, the potential for reduced acute complications and improved long-term outcomes may result in favorable cost-effectiveness ratios over extended time horizons. The development of comprehensive economic models that incorporate quality of life benefits and productivity improvements is essential for informing healthcare policy decisions.
Healthcare system integration challenges include the need for specialized clinical expertise, technical support infrastructure, and data management systems to support artificial pancreas programs. The complexity of these systems requires healthcare organizations to invest in staff training, technical support capabilities, and data analytics infrastructure. The integration with existing diabetes care workflows and electronic health record systems requires careful planning and coordination.
The scalability of artificial pancreas programs represents an important consideration for healthcare system implementation, as the specialized expertise and support requirements may limit the feasibility of widespread deployment. The development of standardized protocols, remote monitoring capabilities, and clinical decision support tools may help address some of these scalability challenges while maintaining quality of care.
Future Technological Innovations and Research Directions
The future of artificial pancreas technology encompasses several promising research directions that could further improve system performance, user experience, and clinical outcomes. Fully automated closed-loop systems that eliminate the need for meal announcements represent a major goal for artificial pancreas development, requiring advances in meal detection algorithms, rapid-acting insulin formulations, and control strategies that can manage large meal-related glucose excursions without prior notification.
Dual-hormone artificial pancreas systems that deliver both insulin and glucagon offer the potential for more physiological glucose control by mimicking both the beta cell and alpha cell functions of the pancreas. Glucagon delivery can provide more effective hypoglycemia prevention and recovery while potentially enabling more aggressive insulin delivery for improved postprandial glucose control. The development of stable glucagon formulations and dual-hormone delivery devices represents an active area of research and development.
Advanced insulin formulations with more rapid onset and offset of action could significantly improve artificial pancreas performance by reducing the pharmacokinetic delays that currently limit system responsiveness. Ultra-rapid-acting insulin analogs and novel delivery approaches such as inhaled insulin may offer improved pharmacokinetic profiles for artificial pancreas applications. The co-formulation of insulin with absorption enhancers or other agents may provide additional opportunities for improving insulin action profiles.
Implantable artificial pancreas systems represent a long-term vision for diabetes technology, offering the potential for more physiological insulin delivery through intraperitoneal or intravascular routes while eliminating many of the challenges associated with subcutaneous systems. The development of biocompatible, long-term implantable devices requires advances in materials science, sensor technology, and surgical techniques. While significant technical challenges remain, implantable systems could provide more effective glucose control with reduced user burden.
Regulatory Considerations and Standards Development
The regulatory pathway for artificial pancreas systems has evolved significantly, with regulatory agencies developing specialized guidance documents and approval processes that address the unique challenges of these complex, integrated medical devices. The FDA has established specific criteria for artificial pancreas systems, including requirements for clinical evidence, safety mechanisms, and post-market surveillance that reflect the sophisticated nature of these systems. The interoperability of system components and the potential for software updates require regulatory frameworks that can accommodate technological evolution while maintaining safety standards.
International harmonization of artificial pancreas regulations represents an important goal for facilitating global development and deployment of these technologies. Differences in regulatory requirements across countries can create barriers to innovation and limit patient access to advanced diabetes technology. The development of common standards and mutual recognition agreements could help streamline the regulatory process while maintaining appropriate safety oversight.
Post-market surveillance requirements for artificial pancreas systems are particularly important given the complexity of these devices and the potential for rare adverse events that may not be apparent in pre-market clinical studies. The collection and analysis of real-world performance data enable regulatory agencies to monitor safety and effectiveness while identifying opportunities for system improvements. The development of standardized data collection and reporting systems facilitates comprehensive post-market surveillance across different device manufacturers and healthcare systems.
The regulation of software updates and algorithm modifications represents a unique challenge for artificial pancreas systems, as these devices may receive periodic software updates that could affect performance and safety characteristics. Regulatory frameworks must balance the need for rapid innovation and improvement with appropriate safety oversight and validation requirements. The development of risk-based approaches to software regulation may help address these challenges while enabling continued technological advancement.
Global Perspectives and Access Considerations
The global deployment of artificial pancreas technology faces significant challenges related to healthcare infrastructure, economic resources, and technical expertise that vary dramatically across different countries and healthcare systems. Developed countries with sophisticated healthcare systems and robust insurance coverage are generally better positioned to adopt artificial pancreas technology, while resource-limited settings may face substantial barriers to implementation. The high cost of these systems, combined with the need for specialized clinical support, creates disparities in access that must be addressed through policy interventions and technology development.
International collaborative research efforts and technology transfer programs can help accelerate the development and deployment of artificial pancreas systems in diverse global settings. Partnerships between developed and developing countries, along with support from international organizations, may help overcome some of the barriers to global access. The adaptation of artificial pancreas technology for different healthcare contexts and resource constraints requires innovative approaches to system design, clinical support, and cost reduction.
The development of simplified, lower-cost artificial pancreas systems specifically designed for resource-limited settings represents an important research and development priority. These systems may incorporate fewer features and require less sophisticated clinical support while still providing meaningful improvements in diabetes control and quality of life. The challenge lies in maintaining safety and effectiveness while reducing complexity and cost.
Training and education programs for healthcare providers in developing countries are essential for successful artificial pancreas implementation, as these systems require specialized knowledge and skills that may not be readily available in all healthcare settings. Distance learning programs, professional exchange initiatives, and technology partnerships can help build the necessary expertise for artificial pancreas care in diverse global contexts.
Ethical and Social Implications
The deployment of artificial pancreas technology raises important ethical considerations related to healthcare equity, patient autonomy, and the medicalization of diabetes management that must be carefully considered as these systems become more widely available. The high cost of artificial pancreas systems creates potential disparities in access that may exacerbate existing healthcare inequities unless addressed through appropriate policy interventions and coverage decisions. The responsibility of healthcare systems and society to ensure equitable access to life-improving diabetes technology represents an important ethical consideration.
Patient autonomy and decision-making capacity represent important considerations in artificial pancreas implementation, as these systems may reduce patient control over diabetes management while potentially improving clinical outcomes. The balance between automated system control and patient preferences requires careful consideration and individualization based on patient values and circumstances. Informed consent processes must ensure that patients understand both the benefits and limitations of artificial pancreas systems.
The psychological and social implications of artificial pancreas technology include both positive effects from reduced diabetes management burden and potential negative effects from increased reliance on medical technology. Some individuals may experience anxiety or loss of control when transitioning from manual diabetes management to automated systems. The development of appropriate psychological support and counseling services may help address these concerns while maximizing the benefits of artificial pancreas technology.
Privacy and data security considerations are particularly important for artificial pancreas systems, as these devices collect and transmit sensitive health information that must be protected from unauthorized access and misuse. The integration of artificial pancreas systems with smartphones, cloud computing platforms, and healthcare information systems creates multiple potential points of vulnerability that must be secured through appropriate technical and administrative safeguards.
Research Frontiers and Emerging Technologies
The convergence of artificial pancreas technology with other emerging healthcare technologies opens exciting possibilities for enhanced diabetes care and management. The integration with digital health platforms, artificial intelligence systems, and precision medicine approaches could enable more personalized and effective diabetes management strategies. Wearable technology and Internet of Things devices may provide additional data streams that could enhance artificial pancreas performance and user experience.
Nanotechnology applications in artificial pancreas systems offer the potential for more sophisticated sensing, drug delivery, and system integration capabilities. Nanoscale glucose sensors, targeted drug delivery systems, and biocompatible materials could enable more effective and less invasive artificial pancreas implementations. The development of implantable nanosystems that could provide long-term glucose monitoring and insulin delivery represents an exciting but distant possibility.
Gene therapy and regenerative medicine approaches may ultimately provide alternative or complementary strategies to artificial pancreas systems, potentially restoring endogenous insulin production through beta cell replacement or regeneration. While these approaches remain largely experimental, they represent important long-term research directions that could fundamentally change diabetes treatment paradigms.
The application of quantum computing and advanced computational methods may enable more sophisticated control algorithms and predictive models for artificial pancreas systems. These computational advances could potentially overcome some of the current limitations in glucose prediction and control optimization while enabling more personalized and adaptive system behavior.
Conclusion
The development of artificial pancreas systems represents one of the most significant advances in diabetes care in recent decades, offering the promise of improved glycemic control, reduced hypoglycemia, and enhanced quality of life for individuals living with diabetes. The successful integration of continuous glucose monitoring, automated insulin delivery, and sophisticated control algorithms has created therapeutic platforms that can provide physiological glucose control with minimal user intervention. While current hybrid closed-loop systems have demonstrated substantial clinical benefits, continued technological advancement promises even greater improvements in diabetes management effectiveness and user experience.
The future trajectory of artificial pancreas development encompasses multiple promising research directions, including fully automated closed-loop systems, dual-hormone delivery platforms, advanced insulin formulations, and implantable devices that could provide more physiological glucose control. The integration of machine learning and artificial intelligence technologies offers the potential for more personalized and adaptive diabetes management that improves over time with accumulated experience. These technological advances must be accompanied by continued attention to safety, accessibility, and healthcare system integration to ensure that the benefits of artificial pancreas technology are realized across diverse patient populations and healthcare settings.
The successful widespread implementation of artificial pancreas technology will require coordinated efforts across multiple domains, including continued technological innovation, supportive regulatory frameworks, appropriate reimbursement policies, and comprehensive healthcare provider education programs. The ultimate goal of artificial pancreas development extends beyond simple glucose control to encompass the broader objective of enabling individuals with diabetes to live full, healthy lives without the constant burden of diabetes self-management. As these systems continue to evolve and improve, they hold the promise of fundamentally transforming the experience of living with diabetes while preventing the acute and chronic complications that have historically defined this challenging condition.
Table 1: Current Commercial Artificial Pancreas Systems Comparison
| System | Manufacturer | Control Algorithm | Key Features | FDA Approval | Age Range |
| MiniMed 780G | Medtronic | Advanced Hybrid Closed-Loop | Auto-correction boluses, smartphone connectivity, predictive low glucose suspend | March 2020 | 7+ years |
| t:slim X2 with Control-IQ | Tandem Diabetes | Predictive hybrid closed-loop | Sleep/exercise modes, smartphone integration, predictive algorithms | January 2020 | 6+ years |
| Omnipod 5 | Insulet | Adaptive hybrid closed-loop | Tubeless design, smartphone control, personalized adaptation | January 2022 | 2+ years |
| CamAPS FX | Cambridge Diabetes Technology | Model Predictive Control | Research platform, meal announcement optional, international availability | CE Mark 2020 | 1+ years (EU) |
Table 2: Artificial Pancreas Control Strategies and Performance Metrics
| Control Strategy | Algorithm Type | Glucose Prediction | Adaptation Capability | Clinical Performance |
| PID Control | Classical feedback control | Limited | Fixed parameters | Time in range 65-70% |
| Model Predictive Control | Model-based predictive | 30-60 minute horizon | Parameter tuning | Time in range 70-75% |
| Fuzzy Logic Control | Rule-based reasoning | Pattern-based | Rule adaptation | Time in range 68-73% |
| Machine Learning | Neural networks/AI | Advanced prediction | Continuous learning | Time in range 75-80% (experimental) |
| Hybrid Approaches | Combined strategies | Multi-modal | Adaptive parameters | Time in range 72-78% |
Table 3: Future Artificial Pancreas Technologies and Development Timeline
| Technology | Development Stage | Expected Timeline | Key Advantages | Major Challenges |
| Fully Automated Closed-Loop | Clinical trials | 3-5 years | No meal announcements required | Meal detection accuracy, insulin speed |
| Dual-Hormone Systems | Early clinical testing | 5-7 years | Enhanced hypoglycemia protection | Glucagon stability, device complexity |
| Implantable Systems | Preclinical research | 10-15 years | Physiological insulin delivery | Biocompatibility, surgical risks |
| Ultra-Rapid Insulin | Clinical development | 2-4 years | Improved postprandial control | Formulation stability, delivery methods |
| Smart Contact Lens Glucose Monitoring | Research phase | 7-10 years | Non-invasive monitoring | Technical feasibility, accuracy |
| Artificial Beta Cells | Early research | 15-20 years | Biological glucose sensing | Engineering complexity, immunogenicity |
 glucosesafe.com
glucosesafe.com